FAT10 - a ubiquitin-like modifier
FAT10 is a ubiquitin-like modifier that is encoded in the major histocompatibility complex and is synergistically inducible by tumor necrosis factor alpha and gamma interferon. It is composed of two ubiquitin-like domains and possesses a free C-terminal diglycine motif that is required for the formation of FAT10 conjugates. (Fig. 1)
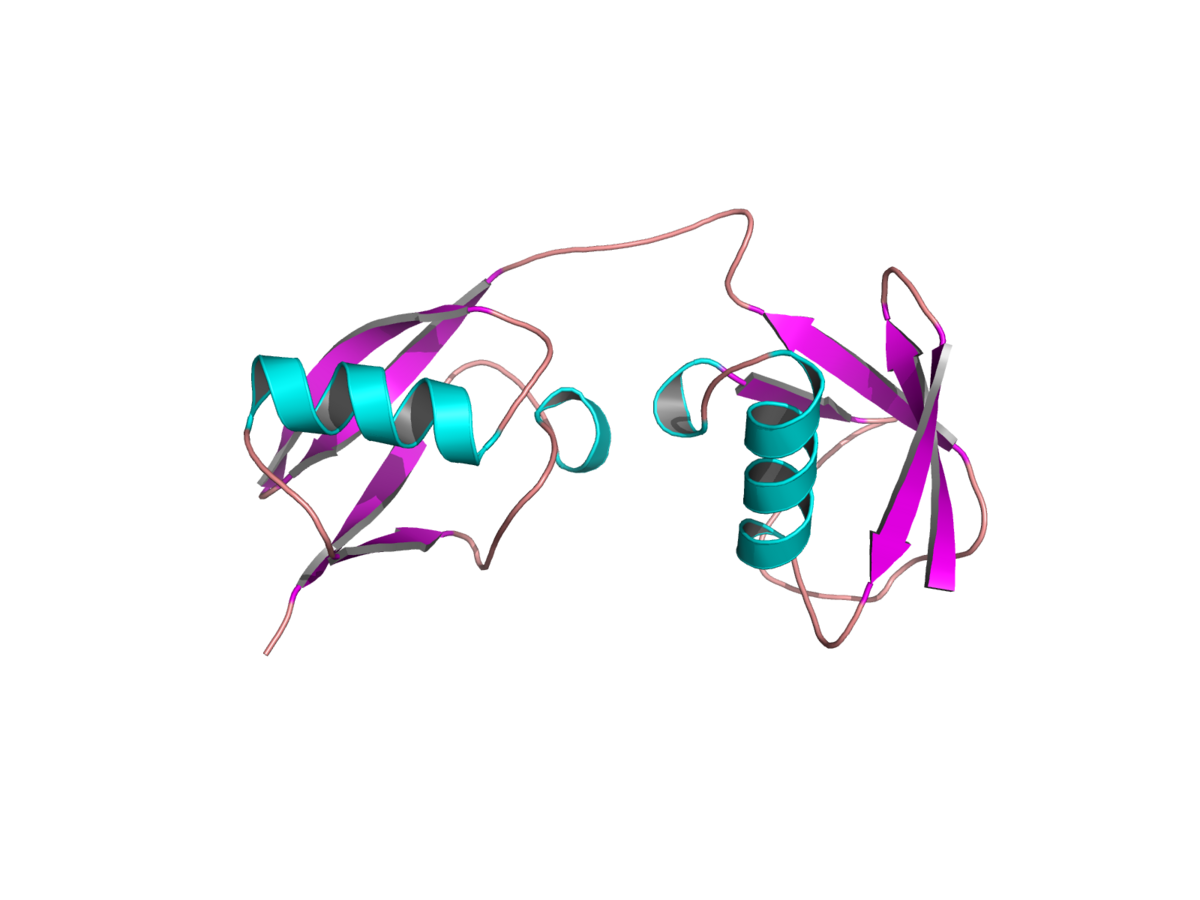
Fig. 1: Model of the structure of FAT10; the two ubiquitin-like domains of FAT10 are connected by a short linker (modified from Groettrup et al. (2008) Trends Biochem Sci 33:230-237).
Like Ubiquitin, which is attached to its substrates via an enzymatic cascade consisting of an activating-(E1), a conjugating-(E2) and a ligating (E3) enzyme, FAT10 forms covalent conjugates to its substrates. We could identify the E1 enzyme (UBA6) and the E2 enzyme (USE1), and currently try to address which E3 supports FAT10 conjugation. Unconjugated FAT10 and a FAT10 conjugate are rapidly degraded by the 26S proteasome at a similar rate. Fusion of FAT10 to the N terminus of very long-lived proteins enhanced their degradation rate as potently as fusion with ubiquitin did. Conjugation with FAT10 is an alternative and ubiquitin-independent targeting mechanism for degradation by the proteasome, which, in contrast to polyubiquitylation, is cytokine inducible and irreversible. Degradation of FAT10 and its conjugates depends on the UBL-UBA-protein NUB1L. So far reported functions of Fat10 include:
- Involvement in dendritic cell (DC) maturation,
- Formation of aggresomes when proteasome is saturated or impaired,
- Mediation of apoptosis in a caspase-dependent manner, especially in renal epithelium and tubular cells
- Mediation of mitotic non-disjunction and chromosome instability
- Mice lacking the FAT10 gene show hypersensitivity to LPS and Extended lifespan and reduced adiposity
Selected readings
- Buerger, S. Herrmann, V.L., Mundt, S. Trautwein, N., Groettrup, M., Basler, M., (2015) - The ubiquitin-like modifier FAT10 is selectively expressed in medullary thymic epithelial cells and modifies T cell selection. J Immunol. 195(9): 4106-16.
- Spinnenhirn, V., Farhan, H., Basler, M., Aichem, A., Canaan, A., Groettrup, M. (2014) - The ubiquitin-like modifier FAT10 decorates autophagy targeted Salmonella and contributes to resistance of mice. J Cell Sci. Nov 15;127(22):4883-93
- Aichem A, Kalveram B, Spinnenhirn V, Kluge K, Catone N, Johansen T, Groettrup M. (2012) - The proteomic analysis of endogenous FAT10 substrates identifies p62/SQSTM1 as a substrate of FAT10ylation. J Cell Sci. 125(Pt 19):4576-85. doi: 10.1242/jcs.107789. Epub 2012 Jul 13.
- Aichem A, Pelzer C, Lukasiak S, Kalveram B, Sheppard PW, Rani N, Schmidtke G, Groettrup M. (2010) - USE1 is a bispecific conjugating enzyme for ubiquitin and FAT10, which FAT10ylates itself in cis. Nat Commun. 1:13. doi: 10.1038/ncomms1012.
- Rani N, Aichem A, Schmidtke G, Kreft SG, Groettrup M. (2012) - FAT10 and NUB1L bind to the VWA domain of Rpn10 and Rpn1 to enable proteasome-mediated proteolysis. Nat Commun. 3:749. doi: 10.1038/ncomms1752.
- Pelzer C, Groettrup M. (2010) - FAT10 : Activated by UBA6 and Functioning in Protein Degradation. Subcell Biochem. 54:238-46. doi: 10.1007/978-1-4419-6676-6_19.
- Schmidtke G, Kalveram B, Groettrup M. (2009) - Degradation of FAT10 by the 26S proteasome is independent of ubiquitylation but relies on NUB1L. FEBS Lett. 583(3):591-4. doi: 10.1016/j.febslet.2009.01.006. Epub 2009 Jan 21.
- Kalveram B, Schmidtke G, Groettrup M. (2008) - The ubiquitin-like modifier FAT10 interacts with HDAC6 and localizes to aggresomes under proteasome inhibition. J Cell Sci. 121(Pt 24):4079-88. doi: 10.1242/jcs.035006. Epub 2008 Nov 25
- Hipp MS, Raasi S, Groettrup M, Schmidtke G. (2004) - NEDD8 ultimate buster-1L interacts with the ubiquitin-like protein FAT10 and accelerates its degradation. J Biol Chem. 279(16):16503-10. Epub 2004 Feb 2
- Hipp MS, Kalveram B, Raasi S, Groettrup M, Schmidtke G. (2005) - FAT10, a ubiquitin-independent signal for proteasomal degradation. Mol Cell Biol. 25(9):3483-91
- Raasi S, Schmidtke G, Groettrup M. (2001) - The ubiquitin-like protein FAT10 forms covalent conjugates and induces apoptosis. J Biol Chem. 276(38):35334-43.
