The Rubisco activation system of diatoms
Alexander Schober
The photosynthetic CO2‐fixing enzyme ribulose 1,5‐bisphosphate carboxylase/oxygenase (RuBisCO) is responsible for the entry of almost all inorganic carbon into the biosphere. In addition to a slow catalytic turnover rate and a wasteful side reaction with oxygen this enzyme forms inhibited complexes with its substrate ribulose 1,5‐bisphosphate (RuBP) and other sugar phosphates. In plants these complexes are removed by the activity of the AAA+ ATPase Rubisco activase (Rca) (Portis 2003). Extensive studies have demonstrated that in plants Rca is a key regulator of photosynthetic carbon fixation and its thermo‐lability is believed to underlie the inhibition of photosynthesis at moderately elevated temperatures (Salvucci and Crafts‐Brandner, 2004).
In contrast the regulation of RuBisCO in diatoms, eukaryotic phytoplankton which is responsible for ~20% of global primary productivity, seems to be different. Diatoms do not possess the rca gene, instead they express two isoforms of an unrelated AAA+ ATPase, the CbbX protein. CbbX has recently been biochemically demonstrated to be a RubisCo activase in the α‐proteobacterium Rhodobacter sphaeroides (Mueller‐Cajar et al., 2011).
We therefore propose to study RuBisCO regulation in the genetically tractable diatom systems Phaeodactylum tricornutum and Thalassiosira pseudonana. Therefore localization and expression studies of the CbbX isoforms will be performed, and CbbX will be characterized under different physiological conditions.
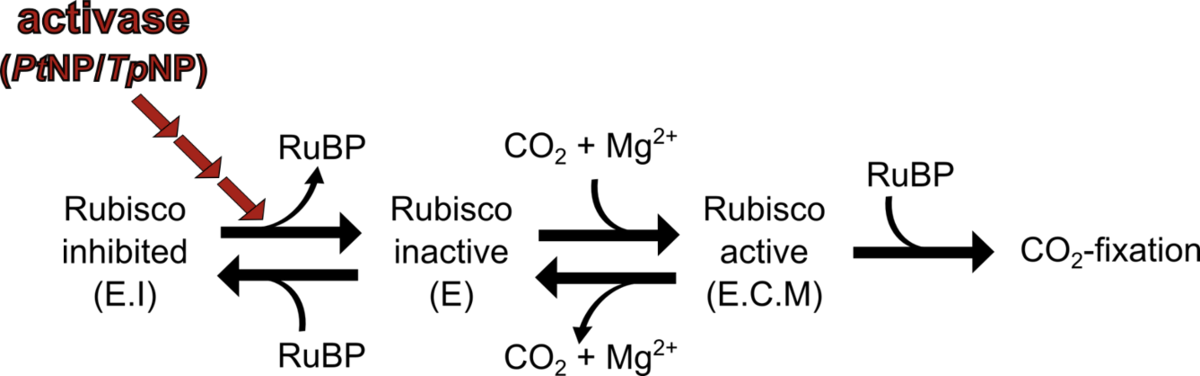
Figure 1: Schematic representation of form I Rubisco activation and inhibition (modified from Mueller-Cajar et al. 2011) Uncarbamylated, inactive enzyme E can bind RuBP to form the inhibited complex E.I or it can react with CO2 and Mg2+ to form the active E.C.M complex. CbbX disrupts E.I and releases the inhibition.
References
Mueller -Cajar, O., M. Stotz, et al. (2011). "Structure and function of the AAA+ protein CbbX, a red-type Rubisco activase." Nature 479: 194-199.
Portis, A. R. (2003). "Rubisco activase - Rubisco's catalytic chaperone." Photosynthesis Research 75(1): 11-27.
Salvucci, M. E. and S. J. Crafts-Brandner (2004). "Mechanism for deactivation of Rubisco under moderate heat stress." Physiologia Plantarum 122(4): 513-519.
