Ubiquitin- and ESCRT-dependent intracellular trafficking in cellular stress responses
Plants are facing constant environmental changes and have to readily respond and adapt to the environment for optimal growth and reproduction. This requires the orchestration of protein synthesis, intracellular transport of proteins, activity regulation of enzymes, and protein degradation.
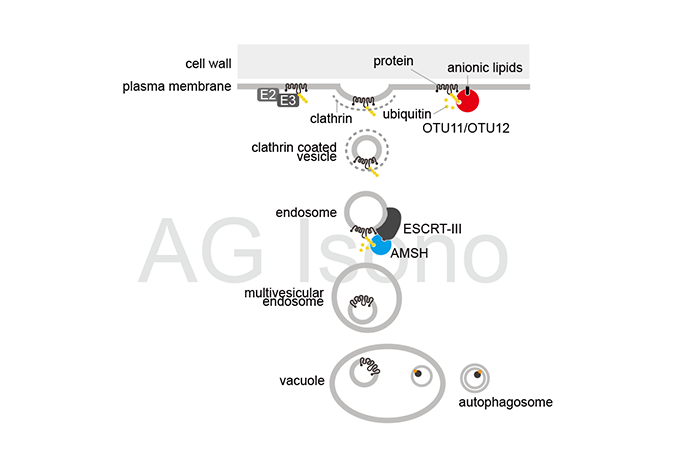
Plasma membrane proteins are crucial for the coordination of the extracellular stimuli and intracellular signaling processes that enable plants to react to environmental changes and stress. Regulation of the abundance of plasma membrane receptors by ubiquitin-and endosomal sorting complexes required for transport (ESCRT)-dependent protein degradation is an important regulatory mechanism in phytohormone signaling pathways as well as biotic- and abiotic stress responses.
Using the model plant Arabidopsis thaliana, we aim to understand the molecular regulation of endosomal and autophagic protein degradation in response to extracellular stimuli. With molecular biological, biochemical and cell biological methods, we address the question as to how posttranslational modification, especially ubiquitylation, is contributing to fine-tuning cellular activities as well as plant growth and development.
Ubiquitination is a process in which ubiquitin - a highly conserved small protein of 76 aa - is covalently attached to target proteins and is, among others, a signal for selective protein degradation. Depending on the modification type, ubiquitylation can serve as a code for numerous different cellular processes.
The conjugation of ubiquitin to its target proteins is primarily mediated by the activity of ubiquitylating enzymes, In addition, deubiquitylating enzymes (DUBs) can also influence the stability of target proteins by removing the ubiquitin molecules from the protein. The spatio-temporal regulation of DUBs is therefore crucial for modulating proteostasis. One aim of our group is to characterize Arabidopsis DUBs that are involved in the regulation of protein stability and to understand their physiological functions.
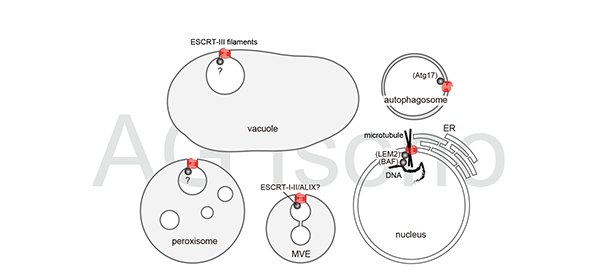
Components of the endosomal sorting complexes required for transport (ESCRTs) are essential for the transport of ubiquitylated membrane proteins. ESCRTs are best known for their function in endosomal trafficking, during which they are required for sorting ubiquitylated membrane proteins into intraluminal vesicles (ILVs) of multivesicular endosomes (MVEs).
ESCRTs have been shown to form filaments of various architecture depending on the subunit compositions and mediate membrane scission and sealing. Although the function of plant ESCRTs has been predominantly discussed in the context of endosomal trafficking, recent studies have revealed a versatile role of ESCRTs in diverse cellular events with broad physiological implications—a process we want to understand at the molecular level.
