Biochemical and physiogical aspects of the ubiquitin-conjugation system
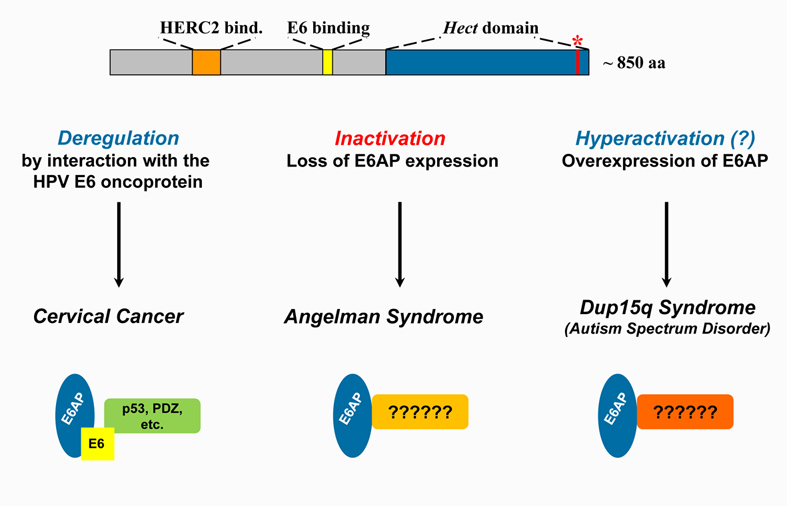
The research program of our group focuses on biochemical and physiological aspects of modification of proteins by ubiquitin ("ubiquitination"), with a special emphasis on the role of the ubiquitin-conjugation system in human disease. Currently, we are studying several ubiquitin-protein ligases including E6AP (encoded by the UBE3A gene), which has been causally implicated in the development of cervical cancer (by virtue of interaction with the HPV E6 oncoprotein), Angelman syndrome (a neurodevelopmental disorder), and more recently, autism spectrum disorders. The results obtained will (hopefully) provide us with intimate insights into the physiological properties/functions of E6AP and how deregulation of E6AP contributes to disease development.
Obtaining insight into how modification by ubiquitin affects the biochemical/physiological properties of a given protein is still a rather challenging task. This is at least in part due to the fact that it is difficult - in many cases impossible - to obtain homogenous and well-defined ubiquitin-protein conjugates from biochemical/biological systems. To overcome this limitation, we have joined efforts with the group of Andreas Marx (Dept. of Chemistry; www.uni-konstanz.de/chemie/~agmarx/) to develop methods that by employing the "unnatural amino acid technology", enable us to generate homogenous ubiquitin conjugates, where ubiquitin or ubiquitin-like proteins are attached to defined residues of a substrate protein .