Research Theme
Insult arising from environmental, occupational, or therapeutic exposure to toxicants, is a primary cause of disease and the treatment of these disorders has significant medical, social and economic implications. Cellular mechanisms directed towards maintaining homeostasis can minimize the negative consequences of environmental stressors; increasing evidence indicates that the outcome of exposure to toxicants results from large networks of molecular and functional changes, occurring across multiple levels of biological organization.
The primary goal of our lab is to elucidate how disease-relevant genetic alterations impact the interplay between epigenetic modifications and gene expression in the context of the cellular stress response, with a specific focus to cancer.
Genes and Environment in Disease
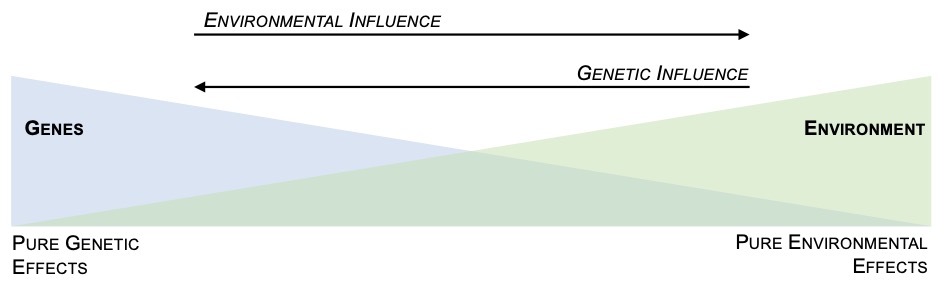
Gene-environment interactions produce gene-regulatory programs that dictate outcome of toxic exposure and disease onset. Specific genetic background (i.e. genetic polymorphisms, hereditary mutations or sporadic premalignant mutations) can influence susceptibility and response to environmental stressors, hence integration of both intrinsic and extrinsic factors determine the cellular, organ and organism response.
The interplay between an individual's genetic makeup, sporadic somatic mutations and environmental exposures is of paramount significance in elucidating the multifaceted processes that underlie the development and progression of cancer.
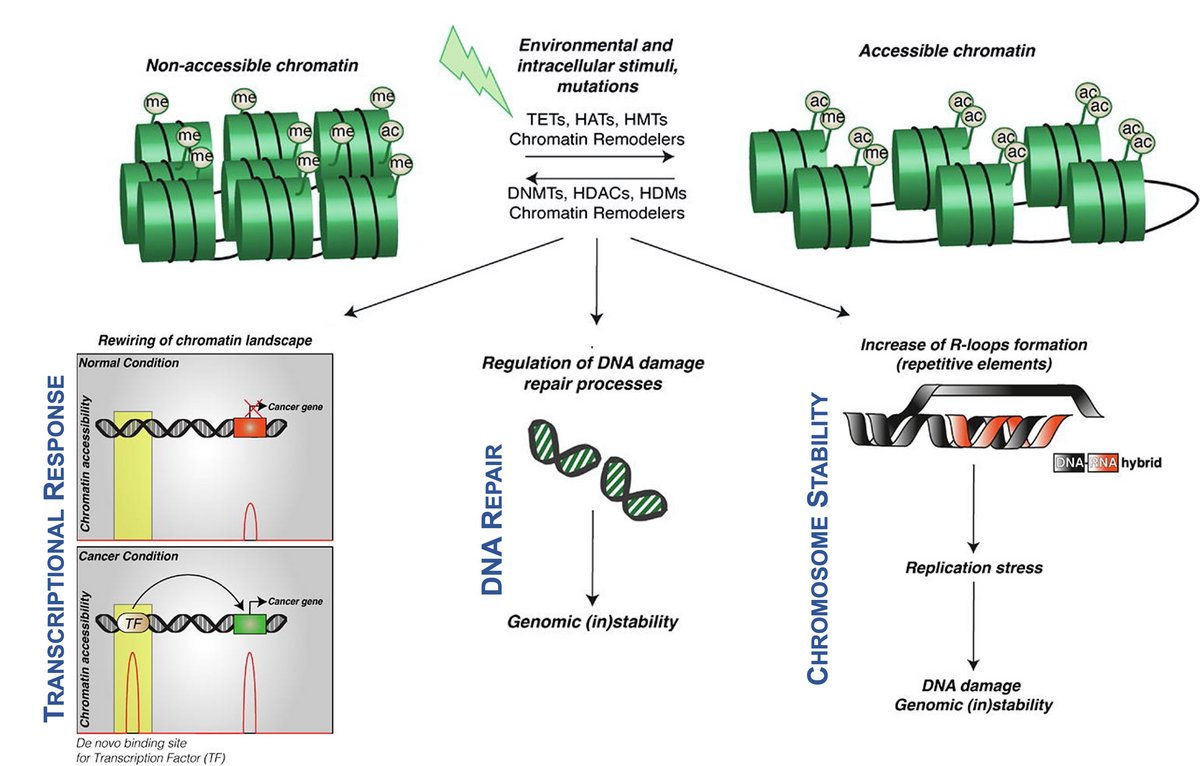
Epigenetic basis of Cell Stress Response
We are particularly interested in the Epigenetic basis of Stress Response. The discovery that exposure to toxicants could produce mutations in the genomic DNA represented a paradigm shift in risk assessment and prevention. The molecular influence of the environmental stressor may however extend well beyond the modification of the genomic DNA, and recent evidence has clearly demonstrated that epigenetic alterations represent a hallmark of environmental insults. Gene-environment interactions can be integrated in the epigenetic code influencing the cellular transcriptome, the DNA repair response and chromosome stability. Identification of the epigenetic basis of these processes provides information on the underlying mechanisms of toxicant-induced disease, such as cancer, enabling identification of early molecular markers of exposure and suggesting potential prevention and/or curative avenues of a negative outcome.