LAS surfactant biodegradation
We aim to uncover the degradation pathway(s) for LAS, the major laundry surfactant in worldwide use (2.5 Mio tons per year). Commercial LAS is a complex mixture of structural homologs and isomers that needs to be degraded in a cooperative effort by very complex bacterial communities, e.g. in sewage treatment systems.
We provided the first bacterial pure cultures that catalyse individual steps of LAS degradation, and these organisms constitute our "model community" on which we study these processes in our laboratory.
Importantly, the complete genome sequence of our model community has kindly been established by the U.S. Department of Energy
Joint Genome Institute (JGI), in particular of Parvibaculum lavamentivorans DS-1, Comamonas testosteroni KF-1 and Delftia acidovorans SPH-1.
The degradation of LAS is accomplished in two tiers, i.e. by first-tier organisms that initially degrade LAS to short-chain sulfophenylcarboxylates (SPC) which are excreted, and by second-tier organisms which mineralize these SPC (see Figure 1 below).
The isolation and characterisation of a representative first-tier organism, Parvibaculum lavamentivorans DS-1 gen. nov. sp. nov., proven to catalyse the conversion of LAS to SPC through omega-oxygenation and fatty-acid beta-oxidation, uncovered that the initial LAS-degradation is more complex than was expected:
(i) commercial LAS is composed of 20 individual congeners (C10 – C13 alkyl chains with different 4-sulfophenyl-substitution positions) and these congeners are degraded by P. lavamentivorans DS-1 into a set of 33 individual major and minor SPCs, and 17 sulfophenyl-di-carboxylates (SPdC). This mixture of SPCs and SPdCs generated by strain DS-1 from commercial LAS is denoted hereafter as SP(d)C.
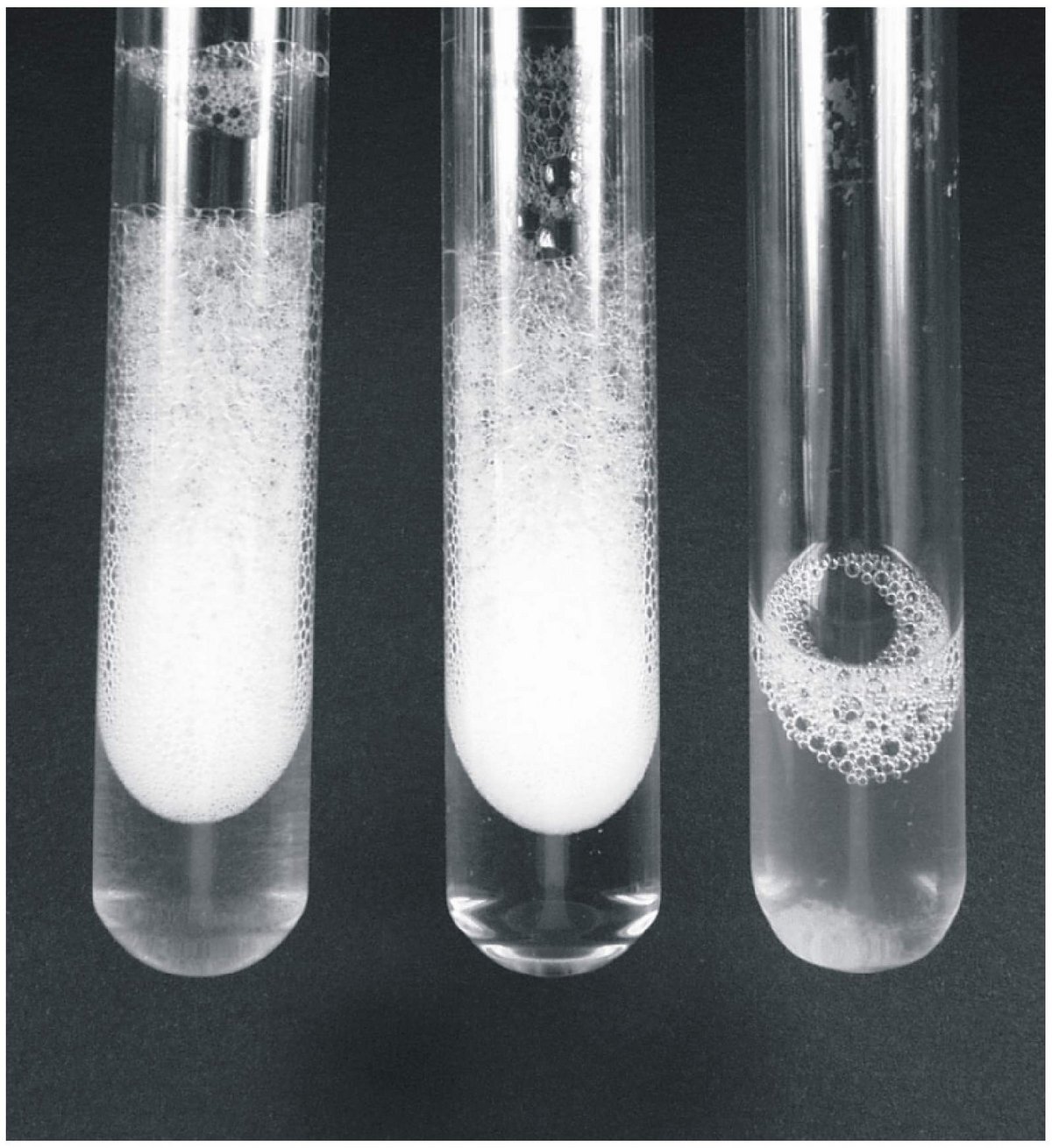
(ii) the growth of strain DS-1 with LAS at high surfactant concentrations is strictly dependent on the presence of a ‘solid support’ in the culture fluid (polyester fleece, glass particles; see Figure 2 below) in conjunction with formation of a biofilm on the solid support; in later growth phases, non-motile cells are also found in suspension. This phenomenon is presumed to be a response of the bacteria to the extended stress in the presence of membrane-toxic surfactants, since growth with a common carbon source (e.g. acetate) is independent of an addition of solid support, and involves no biofilm formation but suspended cells which are motile.
With strain DS-1 available, and with the conditions for growth in LAS-liquid culture determined (addition of solid support), it was possible to generate defined SPC-culture medium after growth of the organism with individual LAS congeners. These SPC-media were used, after removal of the biomass, for the enrichment of representative second-tier organisms from sewage sludge. The isolates obtained, two Comamonas testosteroni strains (SPB-2 and KF-1) and Delftia acidovorans SPH-1, were proven to catalyse the mineralization of individual major SPCs. This work also revealed that the degradation of SP(d)C is more complex than was expected:
(iii) the individual SPC-mineralising isolates have only a very narrow substrate range for SPC (each strain degrading two major SPCs at most), and none of the isolates is able to utilise SPdC. Therefore, a multitude of organisms is needed to degrade all SPCs generated from commercial LAS, to say nothing of the diversity required to degrade all SPdCs. Hence, a very complex and diverse bacterial consortium is involved in degradation of commercial LAS.
(iv) the SPC-mineralising isolates grow in biofilms if surfactant is present in the culture fluid, for example when grown in biofilm-community with P. lavamentivorans DS-1 and with LAS as the sole source of carbon and energy, or when grown in pure culture with a common carbon source (e.g. succinate) in presence of LAS. For the latter condition, the organisms grew on the wall of culture tubes if no solid support was present, in contrast to the growth of P. lavamentivorans DS-1. Hence, the biofilm mode of life is a prerequisite for the maintenance of the community, and for effective LAS degradation.
The isolation and characterisation of these SPC-degrading strains was feasible by the availability of reasonable amounts of individual SPCs, as generated biologically through conversion of individual LAS congeners (see above) provided by industrial sources, or chemically through synthesis using commercially available educts. No other individual LAS congeners, or educts for synthesis, are available.
However, the complex and diverse microbial biofilm community that completely degrades commercial LAS can easily and reproducibly be established in the laboratory, through the use of trickling filters inoculated with biomass from sewage sludge, and when fed with commercial LAS as the sole carbon source for heterotrophic growth. In contrast, apparently all attempts to enrich a community in liquid culture that mineralises commercial LAS over a longer incubation period have failed previously, presumably due to the loss of organisms like P. lavamentivorans during the enrichment. Now, the solid-support dependence of P. lavamentivorans-type organisms is known, and this enables the complex LAS-degrading biofilm community to be grown also in liquid culture on a solid support supplied in the culture medium, e.g. in chemostatic systems.
More simplified still is the new defined three-member community, contributed by LAS-degrading P. lavamentivorans DS-1 and SPC-degrading isolates C. testosteroni KF-1 and D. acidovorans SPH-1, which can also be grown with commercial LAS as three-member biofilm on solid support in liquid culture. This community degrades four major SPCs out of 50 SP(d)Cs, i.e. four major SPCs generated from eight of the 20 LAS congeners.
