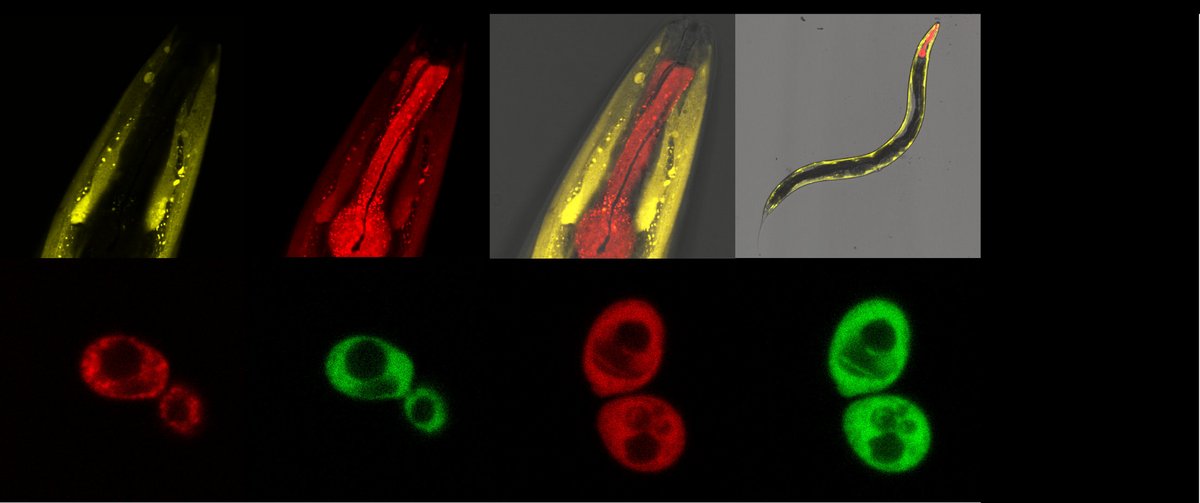
Protein Biogenesis and Molecular Chaperones
The biogenesis of new proteins by ribosomes and their subsequent folding, modification and correct localization to their exact intra- or extracellular compartments are essential key processes of proteostasis to refresh and adapt the proteome. These complex processes are governed by a repertoire of chaperones, i.e. targeting and quality control factors that directly bind to ribosomes near the tunnel exit and work cotranslationally to ensure the correct fate of newly synthesized proteins. In Eukaryotes, two highly conserved chaperone systems bind transiently to ribosomes to ensure proper translation and handling of nascent polypeptide chains. A particular focus is put on studying the functions of the ribosome-associated chaperone systems RAC and NAC (see below) during health, aging and disease conditions. Our goal is to understand the central role of these chaperone systems in proteostasis.
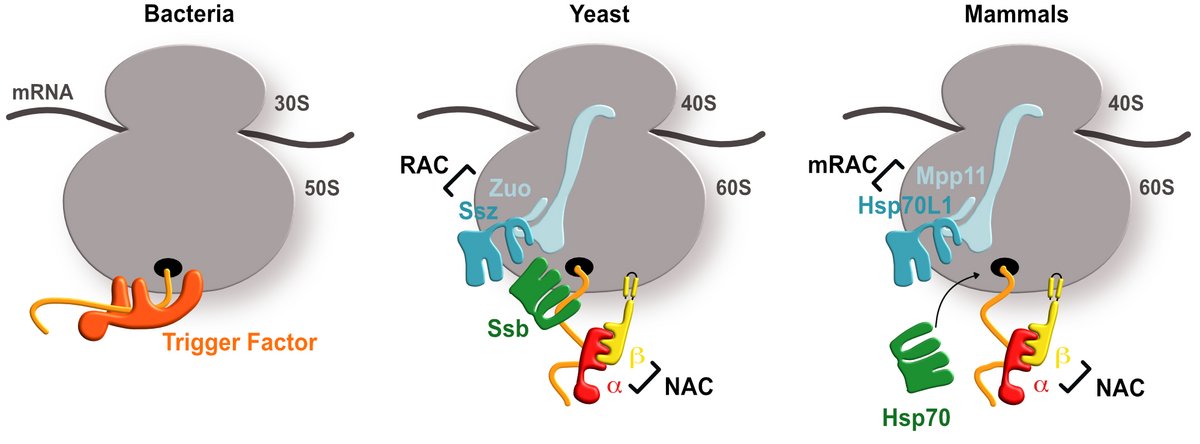
Overview of chaperone systems in bacteria, yeast, and higher eukaryotes
In bacteria, the ribosome-associated chaperone Trigger Factor contacts the nascent chain emerging from the ribosomal exit tunnel. In yeast and higher eukaryotes, the chaperones systems NAC and RAC contact the ribosome and aid in biogenesis of functional proteins.
The Nascent Polypeptide-Associated Complex NAC
(investigated together with Dr. Martin Gamerdinger)
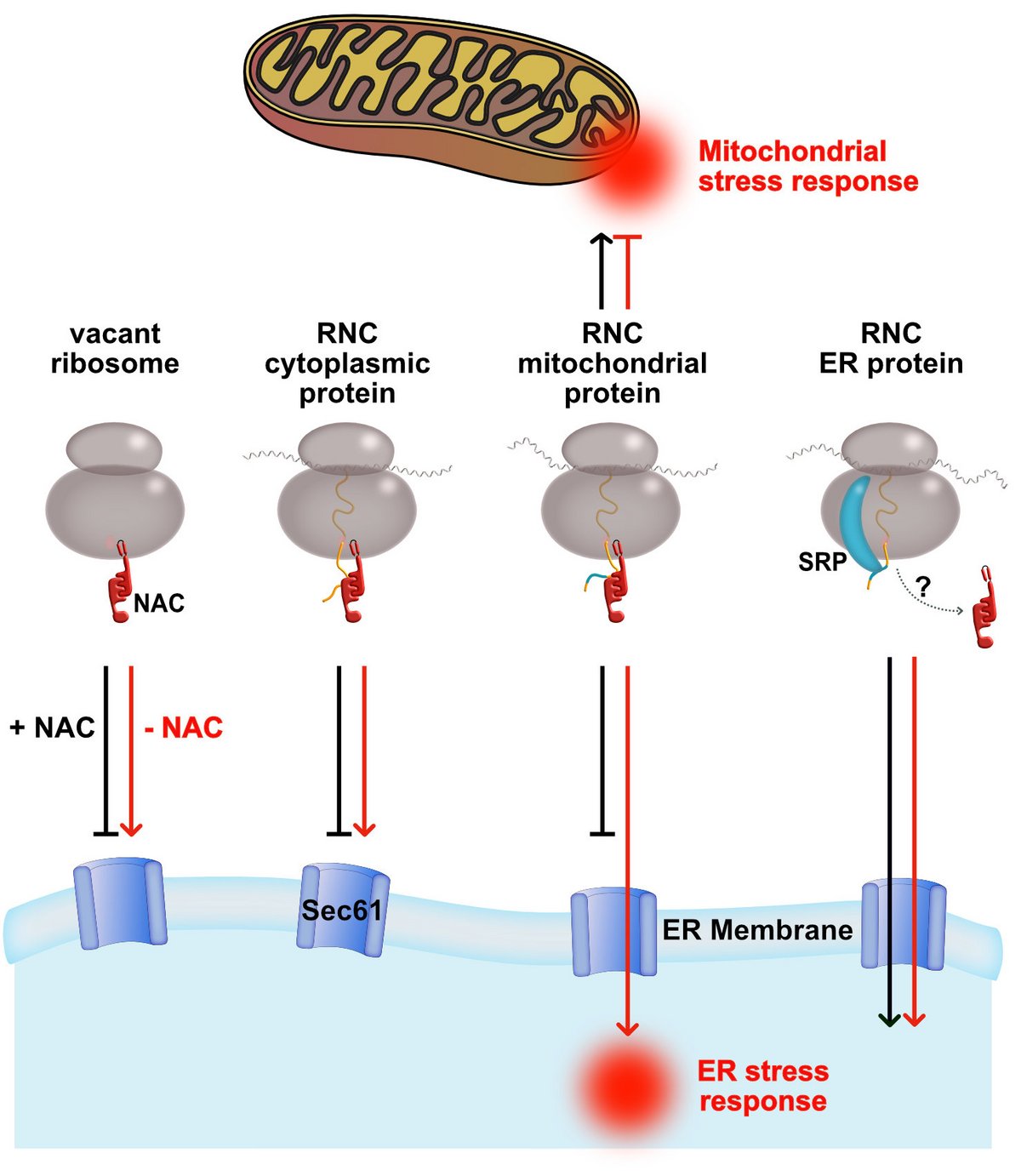
NAC is a highly conserved eukaryotic chaperone complex positioned directly at the ribosomal tunnel exit. It has a central role in guarding the early life of proteins from yeast to man. NAC prevents off-pathway targeting and misfolding events and chaperones nascent polypeptides to the correct biogenesis pathways, thereby guiding them to their native state and final destination.
Schematic representation of the functions of NAC at the ribosomal tunnel exit. Loss of NAC results in mistargeting of nascent polypeptides and leads to mitochondrial and ER stress responses.
Selected Publications
Jomaa, A., Gamerdinger, M., Hsieh, H.-H., Wallisch, A., Chandrasekaran, V., Ulusoy, Z., Scaiola, A., Hegde, R.S., Shan, S.-O., Ban, N., and Deuerling, E. (2022) Mechanism of signal sequence handover from NAC to SRP on ribosomes during ER-protein targeting. Science 375, 839-844 short | full
Gamerdinger, M., Kobayashi, K., Wallisch, A., Kreft, S.G., Sailer, C., Schlömer, R., Sachs, N., Jomaa, A., Stengel, F., Ban, N., and Deuerling, E. (2019) Early scanning of nascent polypeptides inside the ribosomal tunnel by NAC. Mol. Cell 75, 996-1006 short recommended in F1000Prime as being of special significance in its field
Wurmthaler, L.A., Sack, M., Gense, K., Hartig, J., and Gamerdinger, M. (2019) A tetracycline-dependent ribozyme switch allows conditional induction of gene expression in Caenorhabditis elegans. Nat. Commun. 10, 491 short | full
Gamerdinger, M., Hanebuth, M.A., Frickey, T., and Deuerling, E. (2015) The principle of antagonism ensures protein targeting specificity at the endoplasmic reticulum. Science 348, 201-207 short | full
Kirstein-Miles, J., Scior, A., Deuerling, E., and Morimoto RI (2013) The nascent polypeptide associated complex is a key regulator of proteostasis. EMBO J. 32, 1451-1468 short | full
Koplin, A., Preissler, S., Ilina, Y., Koch, M., Scior, A., Erhardt, M., and Deuerling, E. (2010) A dual function for chaperones Ssb/RAC and the NAC nascent polypeptide-associated complex on ribosomes. J. Cell Biol. 189, 57-68 short | full
The ribosome-associated Hsp70/Hsp40 chaperone system RAC
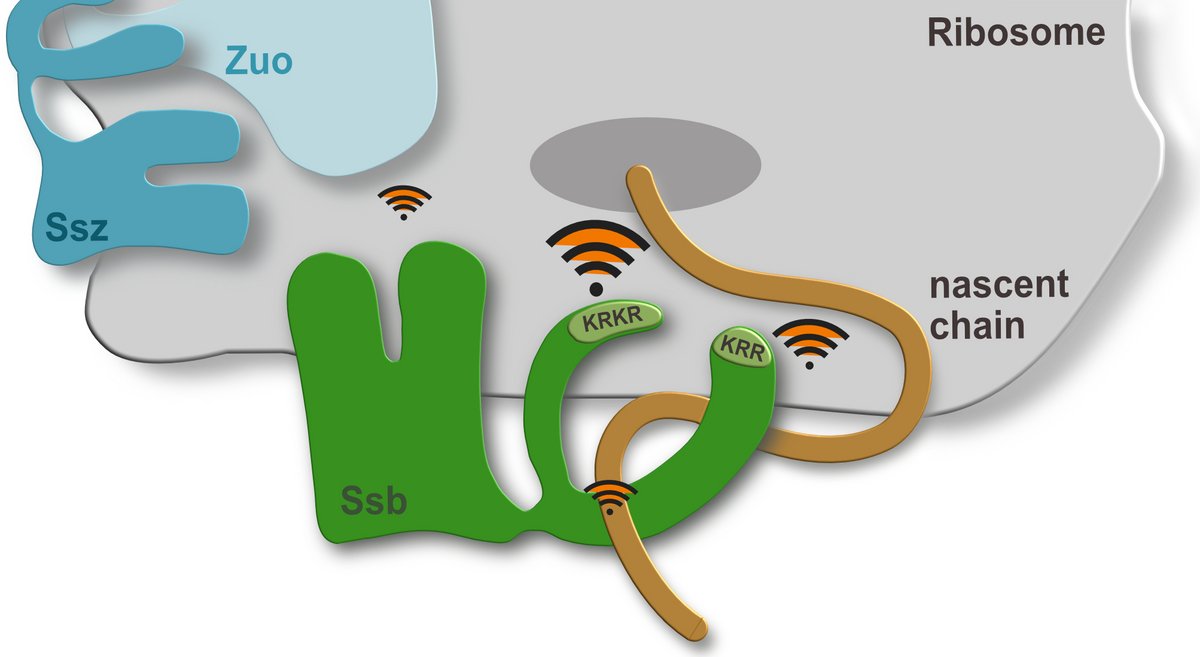
RAC is a stable heterodimer formed by Zuotin (Hsp40) and Ssz (Hsp70). RAC is supposed to regulate translation and to guide early protein folding processes on ribosomes. Zuotin contacts the tunnel exit site on the 60S subunit and spans over the large ribosomal subunit to contact the 40S subunit via an rRNA helix that protrudes into the decoding center of the ribosome. Thus, RAC might affect the conformational dynamics of the ribosome and thereby stall or slow down translation. This led to the hypothesis that polypeptides with folding problems may recruit RAC to regulate their elongation speed and to allow the RAC chaperone system to assist their folding. Yeast RAC is a tripartite system with an additional ribosome-bound Hsp70, Ssb.
The scheme below shows the intrinsic ribosome bindig sites of Ssb (KRKR and KRR, respectively) and two indirect ribosomal contact sites. Taken from Hanebuth et al. 2016
Selected Publications
Fries, S., Braun, T.S., Globisch, C., Peter, C., Drescher, M., and Deuerling, E. (2021) Deciphering molecular details of the RAC–ribosome interaction by EPR spectroscopy. Sci. Rep. 11, 8681 full
Hanebuth, M.A., Kityk, R., Fries, S.J., Jain, A., Kriel, A., Albanese, V., Frickey, T., Peter, C., Mayer, M.P., Frydman, J., and Deuerling, E. (2016) Multivalent contacts of the Hsp70 Ssb contribute to its architecture on ribosomes and nascent chain interaction. Nat. Commun. doi:10.1038/ncomms13695 short | full
Preissler, S., and Deuerling, E. (2012) Ribosome-associated chaperones as key players in proteostasis. Trends Biochem. Sci. 37, 274-283 short | full
Koplin, A., Preissler, S., Ilina, Y., Koch, M., Scior, A., Erhardt, M., and Deuerling, E. (2010) A dual function for chaperones Ssb/RAC and the NAC nascent polypeptide-associated complex on ribosomes. J. Cell Biol. 189, 57-68 short | full
