
Proteostasis Deficiencies: Aging, Stress and Disease
Aging but also other kinds of protein folding stress caused e.g. by inherited mutations or heat shock can lead to dysfunctions in proteostasis andoften to severe diseases and cell death. Also, the loss of chaperone functions can cause severe cellular defects and contributes to the development of protein misfolding diseases such as Creutzfeld-Jakob, Alzheimer´s and Huntington´s disease.
However, we are far from understanding the underlying mechanisms of these deleterious dysfunctions in proteostasis. Thus, our current and future work will focus on chaperones and factors that play a crucial role in these processes during health, disease settings and aging.
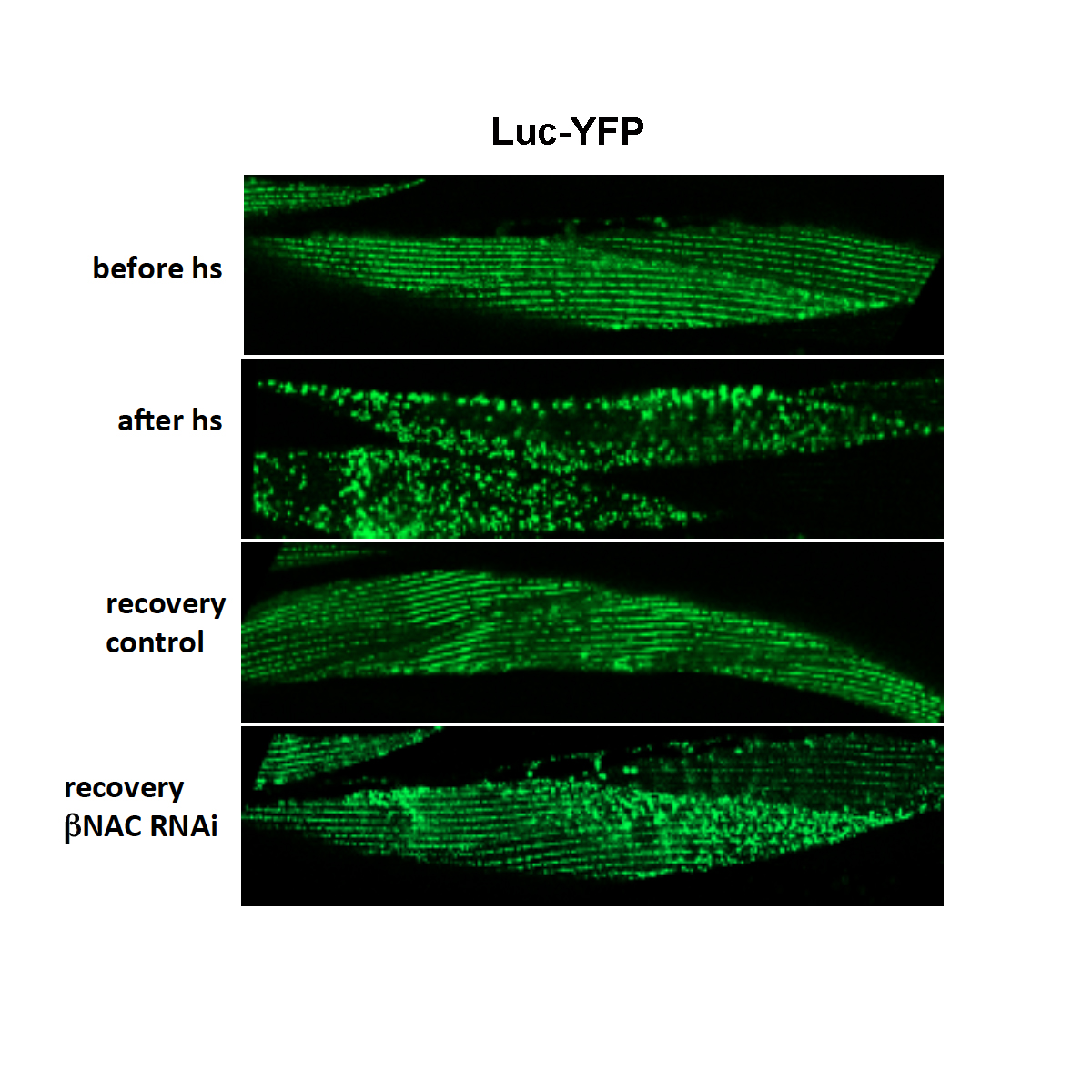
The figure below shows the protein aggregation propensity of luciferase‐YFP expressing animals before heat shock (top), immediately after heat shock (second from top) and after a recovery at 20°C for 24 h in control animals (third from top) or upon knockdown of βNAC during the recovery period (bottom). The scale bars are 10 μm. Taken from Kirstein-Miles et al. (2013)
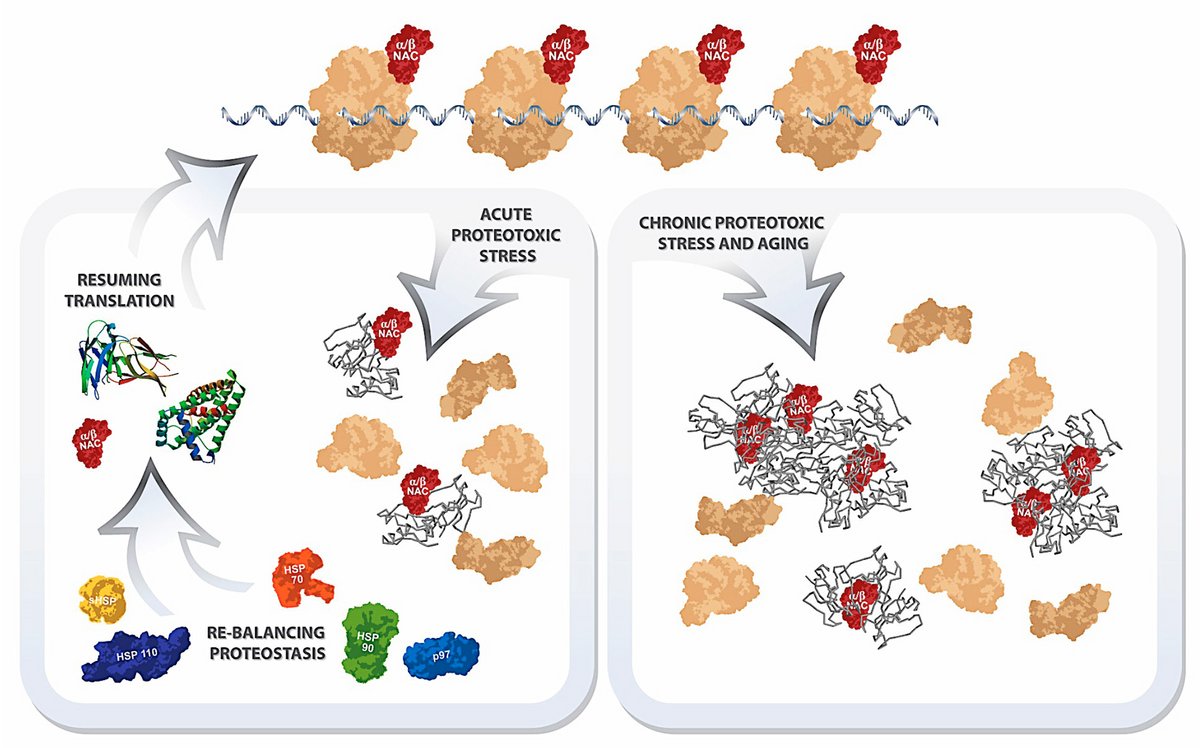
Model for reversible and age/chronic stress‐dependent translational control by NAC. Acute proteotoxic challenges (left branch) lead to a temporary translational attenuation due to a sequestration of NAC by misfolded and aggregated proteins. Re‐balancing of proteostasis liberates NAC and allows for re‐association with ribosomes and translation resumes. Ageing and chronic stress conditions (right branch) lead to a permanent sequestration of NAC and hence to a decrease in protein synthesis. Taken from Kirstein-Miles et al. (2013)
Selected Publications
Shen, K., Gamerdinger, M., Chan, R., Gense, K., Martin, E.M., Sachs, N., Knight, P.D., Schlömer, R., Calabrese, A.N., Stewart, K.L., Leiendecker, L., Baghel, A., Radford, S.E., Frydman, J., and Deuerling, E. (2019) Dual role of ribosome-binding domain of NAC as a potent suppressor of protein aggregation and aging-related proteinopathies. Mol. Cell 74, 729-741 short | full
Wurmthaler, L.A., Sack, M., Gense, K., Hartig, J., and Gamerdinger, M. (2019) A tetracycline-dependent ribozyme switch allows conditional induction of gene expression in Caenorhabditis elegans. Nat. Commun. 10, 491 short | full
Gamerdinger, M., Hanebuth, M.A., Frickey, T., and Deuerling, E. (2015) The principle of antagonism ensures protein targeting specificity at the endoplasmic reticulum. Science 348, 201-207 short | full
Kirstein-Miles, J., Scior, A., Deuerling, E., and Morimoto RI (2013) The nascent polypeptide associated complex is a key regulator of proteostasis. EMBO J. 32, 1451-1468, short | full
Preissler, S., and Deuerling, E. (2012) Ribosome-associated chaperones as key players in proteostasis. Trends Biochem. Sci. 37, 274-283 short | full
