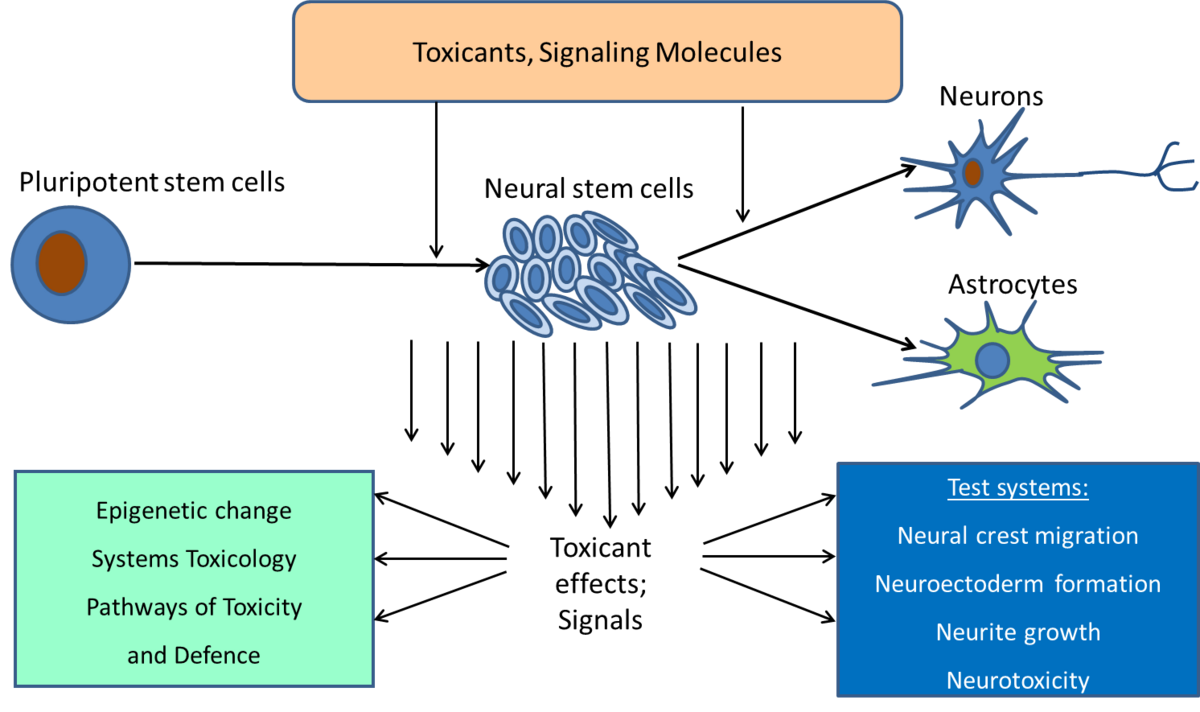
Developmental toxicity
We are interested in how toxicants affect very early embryonic and fetal development. One specialty of the lab is assessment of chemical effects on neural crest cells that are generated from human pluripotent stem cells (ref. 1, 5). Another research line addresses the question on how toxicants affect very early fetal development of the nervous system (--> developmental neurotoxicity). We use pluripotent stem cells that are first differentiated to neural precursors and then further to glial cells or neurons (ref. 7). Using these models of “tissue generation in a cell culture dish”, we examine epigenetic alterations of the cells and how they are affected by toxicants. Moreover, we follow a systems toxicology approach by using gene expression profiling and other techniques that yield rich information on many endpoints, in order to define toxicity signatures of different classes of chemicals and signaling molecules. In our UKN1 test, we measure effects on earliest generation of neural precursors (ref. 2,3,4)
An important mechanism that is afected by toxicants that disturb develop is epigenetic. Especially during embryonic development major chromatin rearrangements are occurring. Whereas a lot is known about epigenetics of mouse development very little is known about early human development. Therefore in our lab we use human Pluripotent Stem Cells (iPSC) and differentiate them into the neuroectodermal lineage to follow the early steps of development, the leaving of the pluripotent state followed by the induction of the germ layer. We are interested in the epigenetic changes during these steps of differentiation and how they are disturbed by toxicants (ref. 6).
Example references:
- Nyffeler J, Dolde X, Krebs A, Pinto-Gil K, Pastor M, Behl M, Waldmann T, Leist M. (2017) Combination of multiple neural crest migration assay to identify environmental toxicants from a proof-of-concept chemical library.
Arch Toxicol, in press - Meganathan K, Jagtap S, Srinivasan S, Wagh V, Hescheler J, Hengstler JG, Sachinidis A*, Leist M* (2015) Neuronal developmental gene and miRNA signatures induced by histone deacetylase inhibitors in human embryonic stem cells.
Cell Death Dis, e1756. doi: 10.1038/cddis.2015.121 - Rempel E*, Hölting L*, Waldmann T, Balmer NV, Schildknecht S, Grinberg M, Das Gaspar JA, Shinde V, Stoebe R, Marchan R, van Thriel C, Liebing J, Meisig J, Blüthgen N, Sachinidis A, Rahnenführer J, Hengstler JG*, Leist M* (2015) A transcriptome-based classifier to identify developmental toxicants by stem cell testing: design, validation and optimization for histone deacetylase inhibitors
Arch Toxicol 89, 1599-1618 - Waldmann T, Rempel E, Balmer NV, König A, Kolde R, Gaspar JA, Henry M, Hescheler J, Sachinidis A, Rahnenführer J, Hengstler* JG, Leist M*, (2014) Design principles of concentration-dependent transcriptome deviations in drug-exposed differentiating stem cells.
Chem Res Toxicol 27, 408-420 - Zimmer B, Lee G, Balmer NV, Meganathan K, Sacchinidis A, Studer L, Leist M (2012). Evaluation of developmental toxicants and signaling pathways in a functional test based on the migration of human neural crest cells.
Env Health Perspect, 120, 1116-1122 - Zimmer B, Kuegler PB, Baudis B, Genewsky A, Tanavde V, Koh W, Tan B, Waldmann T, Kadereit S, Leist M (2011) Coordinated waves of gene expression during neuronal differentiation of embryonic stem cells as basis for novel approaches to developmental neurotoxicity testing.
Cell Death Differ 18, 385-395. - Balmer NV, Weng M, Zimmer B, Ivanova VN, Chambers SM, Nikolaeva E, Jagtap S, Sachinidis A, Hescheler J, Waldmann T, Leist M (2012). Epigenetic changes and disturbed neural development in a human embryonic stem cell-based model relating to the fetal valproate syndrome.
Hum Mol Genet 21, 4104-4114
