New cells and tools
While Craig Venter has opened the race to the development of synthetic cells with new properties, we are on the humble quest of producing known cells from other known cells. Our starting material are stem cells and the target population are e.g. human neurons. Why do we find this interesting? First, as we work on human neurons, we have significantly greater difficulties finding donors for our cell cultures, than laboratories working on blood cells or skin. Second, generation of the desired cells by differentiation allows the study of the differentiation process. We are particularly interested in agents that disturb this process.
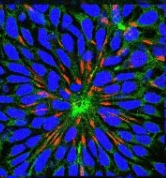
Once, the desired cells are available, a test method has to be built ‘around them’. Important requirements are that it (i) allows a high throughput, (ii) that it behaves in a reproducible way, and (iii) that the endpoint of the test can be quantified. This often requires a lot of technical and bio-informatics development. For instance, we study the capacity of neural stem cells to self-organize in neural rosettes (image above) and the organization of such a rosette is quantified by refined imaging tools. To obtain image data on the rosettes, processes for automated (robotic) microscopy are being developed (high content screening)
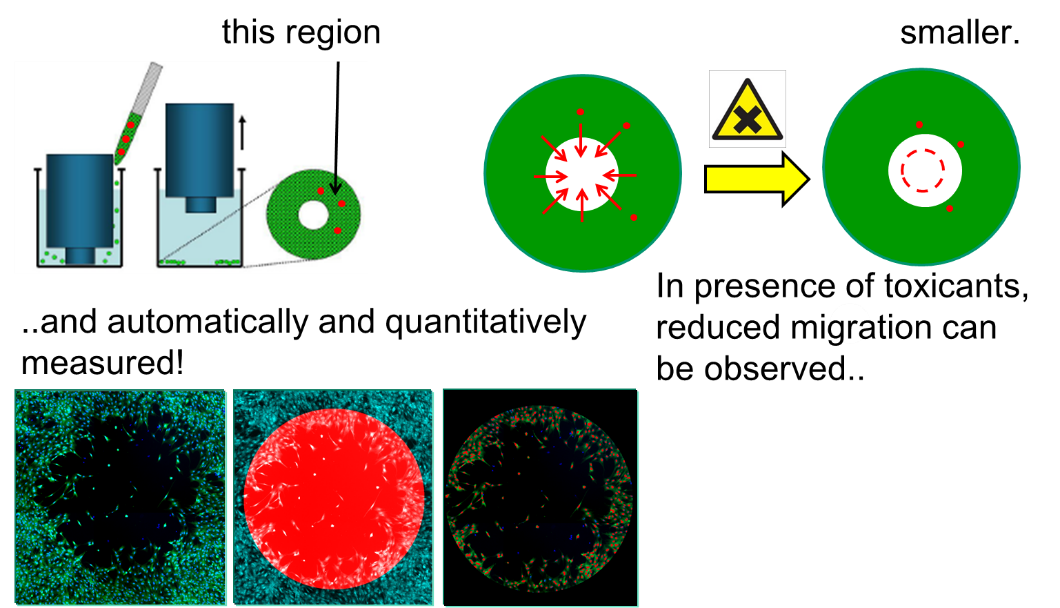
Another example is the high throughput measurement of cell migration, which we perform on neural crest cells (ref. 1 and image below). For the validation of the performance of such assays, calibration tools and standards need to be identified (ref. 2, 3). Besides neural crest cells, we have specialisted in generating astrocytes (ref. 3, 6, 7) and central (ref. 7) or peripheral (ref. 5) neurons.
Example references:
- Nyffeler J, Karreman C, Leisner H, Kim YJ, Lee G, Waldmann T, Leist M (2017) Design of a high-throughput human neural crest cell migration assay to indicate potential developmental toxicants.
Altex 34, 7555-7594 - Aschner M, Ceccatelli S, Daneshian M, Fritsche E, Hasiwa N, Hartung T, Hogberg H, Li A, Mundy WR, Padilla S, Piersma AH, Bal-Price A, Seiler A, Westerink R, Zimmer B, Lein P, Leist M* (2017) Reference compounds for alternative test methods to indicate developmental neurotoxicity (DNT) potential of chemicals: example lists and criteria for their selection and use.
ALTEX 34, 49-74 (R) - Schmidt BZ, Lehmann M, Gutbier S, Nembo E, Noel S, Smirnova L, Forsby A, Hescheler J, Avci HX, Hartung T, Leist M, Kobolák J, Dinnyés A (2017) In vitro acute and developmental neurotoxicity screening: an overview of cellular platforms and high-throughput technical possibilities
Arch Toxicol 34, 1-33 - Chandrasekaran A, Avci HX, Leist M, Kobolak J, Dinnyes A (2016) Astrocyte Differentiation of Human Pluripotent Stem Cells: New Tools for Neurological Disorder Research.
Front Cell Neurosci 10, 215 - Hoelting L, Klima S, Karreman C, Grinberg M, Meisig J, Henry M, Rotshteyn T, Rahnenführer J, Blüthgen N, Sachinidis A, Waldmann T, Leist M (2016) Stem cell-derived immature human dorsal root ganglia neurons to identify peripheral neurotoxicants
Stem Cells Transl Med 5, 476-487 - Kleiderman S, Sá JV, Teixeira AP, Brito C, Gutbier S, Evje LG, Hadera MG, Glaab E, Henry M, Agapios S, Alves PM, Sonnewald U, Leist M (2016) Functional and phenotypic differences of pure populations of stem cell-derived astrocytes and neuronal precursor cells
Glia 64, 695-715 - Schildknecht S, Kirner S, Henn A, Gasparic K, Pape R, Efremova L, Maier O, Fischer R, Leist M (2012). Characterization of mouse cell line IMA2.1 as a potential model system to study astrocyte functions.
ALTEX 29, 261-274 - Scholz D, Pöltl D, Genewsky A, Weng M, Waldmann T, Schildknecht S, Leist M (2011) Rapid, complete and large-scale generation of post-mitotic neurons from the human LUHMES cell line.
J Neurochem 119, 957-971
